CO Stripping with EC-MS: Advanced Catalyst Analysis by Spectro Inlets
EC‐MS Application Note #1
Summary of the Article
The article titled “Application Note 01: CO Stripping” by Spectro Inlets details the process and importance of CO stripping in electrochemical measurements. It focuses on the use of Electrochemical Mass Spectrometry (EC-MS) to analyze and quantify the electrochemical oxidation of carbon monoxide (CO) on catalyst surfaces, particularly platinum. The document outlines the experimental setup, the procedure for CO stripping, and the significance of the data obtained.
Interested?
Do not hesitate to contact us for a quotation or a talk and presentation of our product
Key Points:
CO Stripping Process: CO stripping is a technique used to study the electrochemical activity of catalysts. CO is adsorbed onto the catalyst surface, and then the potential is increased to oxidize the CO, allowing for the measurement of the resulting current.
Experimental Setup: The setup includes an electrochemical cell connected to a mass spectrometer. The system is designed to measure the products of the CO oxidation reaction in real-time.
Procedure: The procedure involves purging the electrochemical cell with inert gas, introducing CO to adsorb on the catalyst, and then stripping the CO by applying a potential sweep. The resulting current and evolved gases are monitored.
Data Analysis: The mass spectrometer detects the gases evolved during the oxidation process, providing detailed information about the reaction kinetics and the efficiency of the catalyst.
Applications: This technique is crucial for developing and testing catalysts for fuel cells and other electrochemical devices. It provides insights into the catalyst’s behavior and helps in improving its performance.
Relevance to EC-MS from Spectro Inlets
The relevance of CO stripping to the EC-MS system developed by Spectro Inlets can be broken down into several key aspects:
Enhanced Catalyst Evaluation: The EC-MS system enables precise monitoring of the gases evolved during CO stripping. This is essential for evaluating the performance and efficiency of catalysts, particularly in fuel cell applications.
Real-Time Analysis: The ability to perform real-time analysis of gas evolution during electrochemical reactions provides immediate insights into the reaction mechanisms. This is crucial for understanding and optimizing catalyst behavior.
High Sensitivity and Accuracy: The mass spectrometer used in the EC-MS system offers high sensitivity and accuracy in detecting minute quantities of gases. This enhances the reliability of the data obtained from CO stripping experiments.
Comprehensive Data: Combining electrochemical measurements with mass spectrometric analysis allows for a comprehensive understanding of both the electrochemical and gas evolution aspects of the reaction. This dual approach is beneficial for developing more efficient catalysts.
Versatile Applications: While the article focuses on CO stripping, the EC-MS system is versatile and can be used for a variety of electrochemical studies. This makes it a valuable tool for researchers working on different types of electrochemical devices and reactions.
Overall, the EC-MS system from Spectro Inlets provides a powerful and precise method for conducting CO stripping experiments, which are vital for advancing the development of electrochemical catalysts and devices.
CO‐stripping Technique
EC‐MS Application Note #1
last updated 05‐01‐2022
Why Do a CO‐strip?
The CO‐strip technique is based on the electrochemical oxidation of an adsorbed layer of CO from a sample surface of interest. The technique is often used to quantify the active surface area of the sample and can provide insight on crystalline facets and binding energies of the adsorbed species. Furthermore, the CO2 mass spectrometer (MS) signal of the ECMS may be calibrated via the CO‐strip technique.
∗CO + H2O −→ CO2 + 2 H+ + 2 e− (1)
The overall reaction is given by eq. (1), where a CO molecule adsorbed on a surface site (∗CO) is oxidized to CO2 with a net transfer of two electrons. Thereby the total charge passed during a CO‐strip is a direct measure of the total number of sites present on the studied surface. At the same time, the released CO2 is detected in the MS. Again according to eq. (1), the amount of CO2 released in the strip can be related to the total charge passed during the CO strip, which can be used to calibrate the CO2 signal. This application note, will demonstrate how to perform a CO‐strip experiment using the EC‐MS system and the key steps of the data treatment procedure.
Experimental Procedure for CO‐strip
To successfully carry out a CO‐strip experiment, the sample studied should bind CO sufficiently strong, e.g. Pt or Pt‐group metals. The general experimental procedure follows three main steps (i‐iii).
(i) Reference measurement
(ii) CO‐poisoning
(iii) CO‐stripping
First, a stable CV under inert gas saturation should be obtained as a reference measurement (i) for verification and as a baseline. Next, the gas is changed to CO, which poisons the sample surface (ii) while the potential is held constant at a value where CO is not oxidized. The inert gas is switched back on and remaining non‐adsorbed CO is purged out. Then, the CO is stripped of (iii) in an anodic‐going sweep in a CV, giving rise to a faradaic current. Finally, the reference measurement from step (i) is repeated. There are many parameters which may influence the CO‐strip experiment, such as electrode material, surface structure,
electrolyte, adsorption potential, scan speed etc. In the following example a typical COstrip experiment using a polycrystalline Pt disk in 0.5 M H2SO4 is shown, using parameters that are suitable for this system. The parameters used here might not be optimal for other systems.
First, a few points regarding safety and preparation of the experiment are discussed. Next, the experimental procedure with the EC‐MS is described, followed by the data treatment procedure.
Safety & Experimental Considerations
Before starting a CO‐strip, make sure that a completely dry and rigorously cleaned cell is available, a chip is installed in the setup, and electrolyte, sample and other accessories are prepared. Furthermore, a make‐up gas line (e.g. He) and a CO line need to be connected to the EC‐MS. Always pump down and purge newly installed gas lines several times to minimize contamination of gas supply and the EC‐MS.
Precautions need to be taken when working with CO and other toxic, flammable or otherwise hazardous compounds. Avoid any leak or outlet of CO and ensure proper ventilation of the work area. The use of a gas alarm or a handheld CO‐detector is advisable. During experiments, the gas outlet of the EC‐MS setup (roughing pump outlet) should be led into a ventilation point to avoid the release of CO in the work area. Prepare a risk assessment according to your institution’s rules before starting any experiment involving CO.
Metal (e.g. Ni, Fe) carbonyls may form by exposing a metal (e.g. steel) to CO at a high pressure. The decomposition of the metal carbonyl at the electrode can lead to contamination mand may cause unwanted effects in experiments. Several steps can be taken to minimize the effect:
- Keep the pressure in the CO supply line low to minimize the driving force towards
forming metal carbonyls - Install a carbonyl filter at the end of the CO line just before entering the EC‐MS
- Avoid nickel containing fittings and tubing to the extent possible
- Flush the CO line to remove stagnant gas before starting an experiment
Reference Measurement
Start by setting up experimental parameters in Zilien and EC‐lab. The experimental procedure in EC‐lab should contain a CVA technique followed by a loop. Choose suitable values of parameters such as current range, bandwidth, scan rate etc. depending on the experiment and system studied. For further detail on EC parameters consult the EC‐lab manual and application notes, as well as EC‐MS technical note #3. Pump down the chip and start the Zilien experiment. Set a flow of 1 mL/min inert make‐up gas (e.g. He) on line #1 via MFC1. Prior to starting EC experiments, perform a breath test and a drop test to verify that the EC‐MS is fully operational. Mount cell with sample, inject electrolyte and connect glass pipes and electrode connections. For more details on preparation steps above, see the EC‐MS manual. Make a reference measurement:
(i) Trigger the EC experiment from Zilien and wait to obtain a stable CV. Use the same set of parameters in EC‐lab that will be used during the CO‐strip for comparison (scan speed, potential limits etc.).
(ii) Obtain the reference measurement: First, end the CV with a potential hold at +0.05 V vs. RHE for 10 min.
(iii) Then start cycling the potential again (using the loop to restart the CVA technique). The first cycle can be used as a baseline for background subtraction during data treatment.
Gas Exchange to Induce CO‐poisoning
Next, CO is introduced to the cell to induce CO‐poisoning. Keep the same Zilien and EClab measurements running that were used for the baseline measurement. Before switching gases, ensure the inlet valve on the CO line is open (i.e. V9 when line #2 is used for CO) and the MFC2 mode is normal. Now perform the gas exchange:
(i) End the CV with a potential hold at +0.05 V vs. RHE)
(ii) Write 10 ml/min in the MFC2 setpoint field but do not press enter or click outside the field.
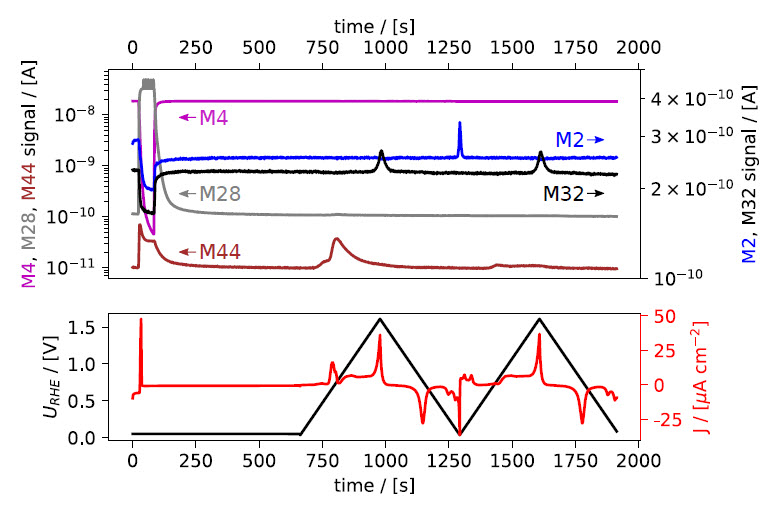
(iii) In quick succession click V8 and write and enter 0 mL/min in the MFC1 setpoint field. This connects line #2 to the chip while initializing a 10 ml/min flow of CO, while line #1 is closed and the He flow is stopped. Now CO enters the chip and saturates the electrolyte. The M28 signal seen in the MID will promptly increase while M4 decreases, as seen in the top panel of Fig. 1a. Simultaneously, a peak is observed in the current (red trace, bottom panel) due to CO displacing adsorbed hydrogen on the Pt electrode.
(iv) When the M28 signal stabilizes at a maximum, the make‐up gas can be switched back to the inert gas: write but do not enter 10 mL/min in the MFC1 setpoint field. In quick succession click on V8 and write and enter 0 mL/min in the MFC2 setpoint field.
(v) Keep an eye on the pressure at P3 when running gas flows at 10 mL/min. If P3 is higher than expected, the gas ballast on the scroll pump should be used. After a few minutes the He flow can be turned down to 1 mL/min.
(vi) If the CO gas is no longer required in this measurement the MFC2 mode can be set to Flow Off and the inlet valve closed (e.g. V9 when line #2 is used for CO). If instead a different gas is desired on line #2, the entire line (all the way through MFC2) should be pumped via the gas manifold.
(a) EC‐MS plot during gas exchange and CO‐strip as a function of time
(b) CO stripping CVs as a function of potential.
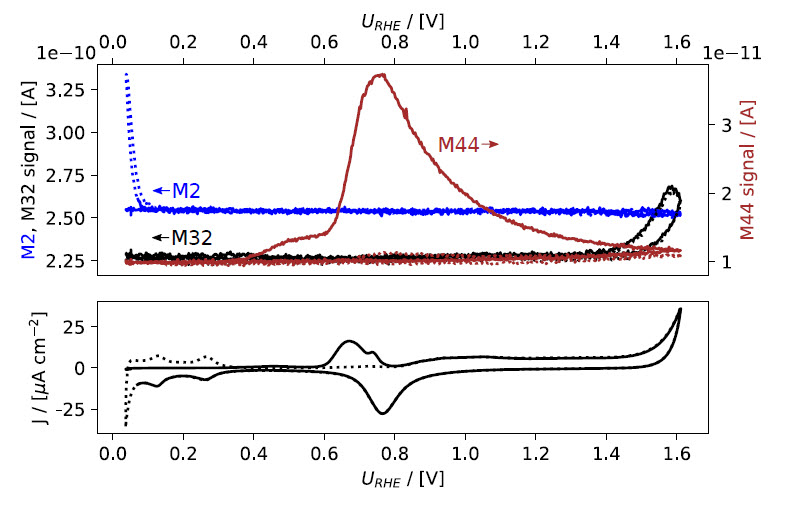
Figure 1: EC‐MS plot during gas exchange and CO‐strip. Mass spectrometer signals are shown in the top panels, electrochemical potential vs. RHE and current density in the bottom panels. ﴾a﴿ Initially, the potential is held at +50 mV vs. RHE while the make‐up gas is changed from He to CO, as seen in the M4 and M28 signals. A sharp peak in the current density (red) is observed immediately, due to CO displacing adsorbed hydrogen on the Pt sample. The potential is held constant while switching the make‐up gas back to He until all non‐adsorbed CO is purged out. Upon sweeping the potential ﴾5 mV/s scan rate﴿ anodically, a peak in the
electrochemical current and the M44 signal are observed, indicating the oxidation of adsorbed CO to CO2. (b) shows the CVs as a function of potential. The second cycle from (a) is shown as baseline (dotted).
CO Strip
After switching back to inert make‐up gas (e.g. He), wait for the M28 signal to drop back to baseline level, to ensure that all non‐adsorbed CO has been removed from the gas lines and the sample volume. The total duration of the potential hold period should be the same as for the reference measurement. Then continue the CV in EC‐lab, in the same manner as for the reference measurement. This time, an oxidation peak can be observed in the electrochemical measurement, concurrently with a peak in M44, see Figs. 1a and 1b. Choose a low scanrate (e.g. 5 mV/s or lower) for optimal time response of the MS signal.
Data Treatment
The following section illustrates the key steps of data analysis. Note that there is a vast amount of scientific literature discussing how to use CO‐stripping to determine surface area. The analysis shown below serves as an example in the context of EC‐MS, and we refer the reader to the scientific literature for further details[1–3]. Using CO‐stripping for MS calibration, is of course limited to the calibration of the CO2 signal.
Surface Area Determination
Assuming ideal behavior, adsorbed CO forms a monolayer coverage on the surface. The faradaic current passed when oxidizing this monolayer can therefore be used to determine the number of surface sites available for CO adsorption, which gives an estimate for the electrochemically active surface area (ECSA). As the density of atoms on the surface depends on the exposed facet, and the coverage is not always a full monolayer, usually conversion factors from the literature are used to convert the faradaic charge to surface area. Here, we use a factor of 340 μC cm– 2 for polycrystalline Pt. The faradaic charge is determined by integrating the stripping current with respect to time, between 0.4 and 0.9 V vs. RHE. As the oxidation of CO occurs in a similar potential range, as the onset of Pt surface oxidation, the charge determined from a reference measurement in the absence of CO is subtracted, see eq. 2 and Fig. 2.
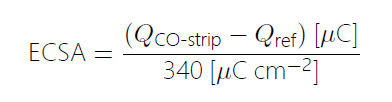
(2)
In the measurement shown in Fig. 2, using the second cycle as reference, an ECSA of 0.208 cm2 was determined, or 0.193 cm2 when using the baseline cycle determined in the reference measurement. For comparison, the geometric surface area of the electrode used is 0.196 cm2.
CO2 Calibration
As mentioned, the faradaic current observed during the CO‐strip is related to the number of molecules of CO being oxidized according to Faraday’s law of electrolysis, where Q is the total charge, z the number of electrons in the reaction (z=2 in the case of CO oxidation) and F the Faraday constant:
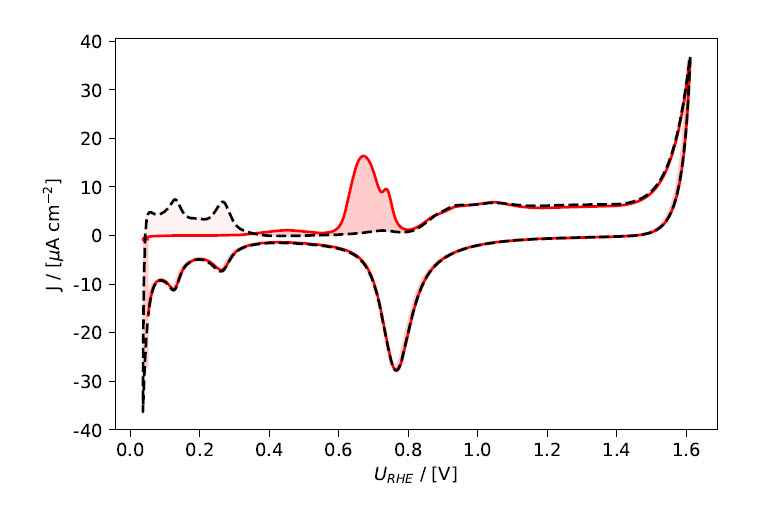
Figure 2: Electrochemical current vs. potential: The red, full line shows the CO stripping cycle, the black, dashed line the second cycle used as reference cycle. Both cycles were integrated in the potential range from 0.4 to 0.9 V vs. RHE on the anodic scan, the difference is highlighted by the darker red shading in the figure.
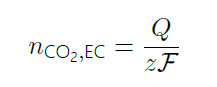
(3)
Due to the design of the EC‐MS chip, all CO2 formed during the CO strip will be observed in the MS, which means that the faradaic current can be used to calibrate the MS signal of CO2 to convert from detector current IMS in [A] to molecular flux n˙ CO2 in [mol/s]:
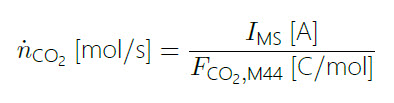
(4)
Where FCO2,M44 is the calibration factor for CO2 measured at m/z=44 (M44), according to eq. 5:
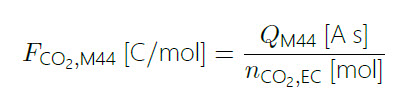
(5)
The integrated M44 signal is shown in Fig. 3. Also in this case it is important to subtract a background signal, both when determining the amount of CO2 released from the faradaic current, and when integrating the M44 signal from the MS. Either a separate measurement without introducing CO, or the CO2 released in the second cycle after dosing CO can be used as such a background signal. In either case, trace carbon contamination might contribute to the M44 background. In the example shown here, a calibration factor FCO2,M44 of 5.1 C/mol was determined when using the baseline measurement without CO as a reference. Note that this calibration factor depends on the tuning of the MS and which detector is being used (Faraday cup or electron multiplier) and therefore needs to be determined regularly and can deviate from the value stated here.
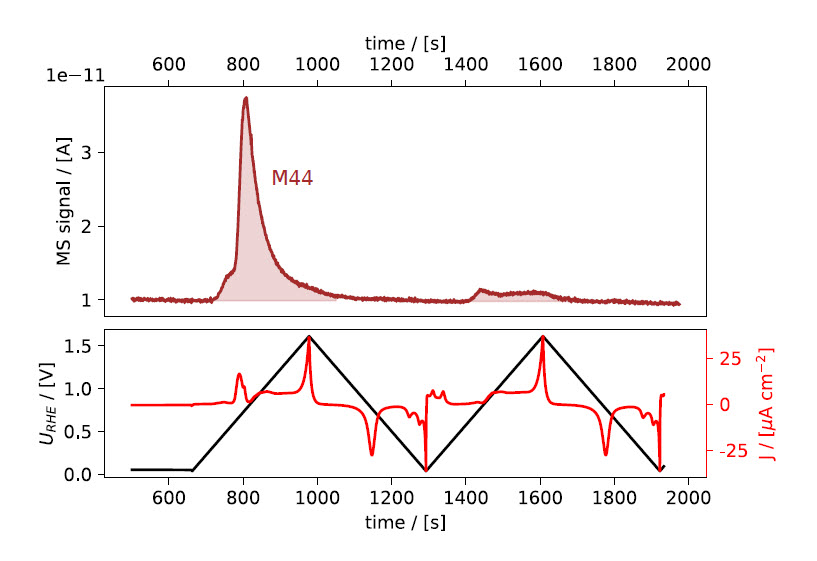
Figure 3: EC‐MS plot highlighting the integrated CO2 signal from the CO‐strip shown in Fig. 1a Note that the baseline is not at 0 A, therefore a suitable baseline level should be chosen for the integration.
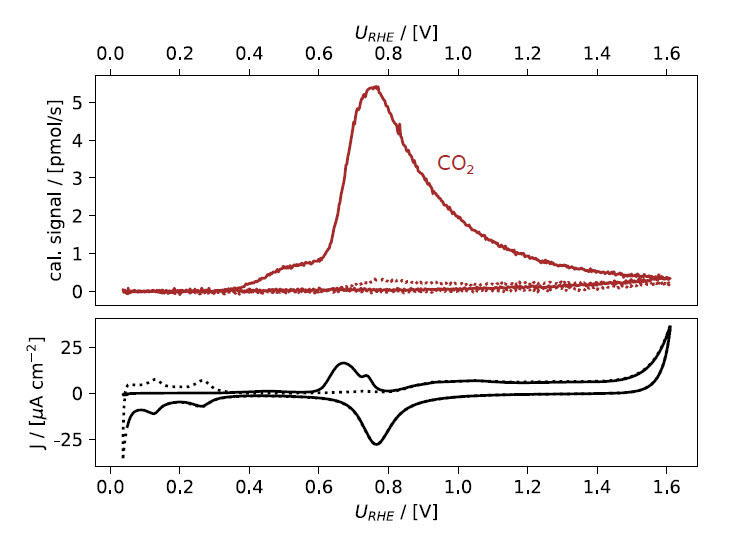
Figure 4: EC‐MS plot as shown in Fig. 1b after calibration of the M44 signal to show the molecular flux of CO2 released in CO strip. In addition to calibration, the signal was normalized to the baseline.
Using the calibration factor, the raw M44 signal can then be plotted as CO2 flux instead, as shown in Fig. 4. This figure highlights the high sensitivity of the EC‐MS, where a mass flux on the order of only a few pmol/s can be followed in real time.
References
[1] S. Rudi, C. Cui, L. Gan, and P. Strasser, “Comparative Study of the Electrocatalytically Active surface areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and COstripping Voltammetry,” Electrocatalysis, vol. 5, pp. 408–418, 4 2014. DOI: 10.1007/s12678-014-0205-2.
[2] A. Cuesta, A. Couto, A. Rincón, M. Pérez, A. López‐Cudero, and C. Gutiérrez, “Potential Dependence of the Saturation CO Coverage of Pt Electrodes: The Origin of the Pre‐peak in CO‐stripping Voltammograms. Part 3: Pt(poly),” Journal of Electroanalytical Chemistry, vol. 586, pp. 184–195, 2 2006. DOI: 10.1016/j.jelechem.2005.10.006.
[3] T. Binninger, E. Fabbri, R. Kötz, and T. J. Schmidt, “Determination of the Electrochemically Active Surface Area of Metal‐Oxide Supported Platinum Catalyst,” Journal of The Electrochemical Society, vol. 161, H121–H128, 3 2014. DOI: 10.1149/2.055403jes.
All data treatment and plotting in this application note was carried out using the open source Python package ixdat, available at https://github.com/ixdat/ixdat.
Interested?
Do not hesitate to contact us for a quotation or a talk and presentation of our product
