Publications
FIND PEER-REVIEVED PUBLICATIONS, RESEARCH PAPERS, SCIENTIFIC ARTICLES OR ACADEMIC JOURNALS RELATED TO ELECTROCHEMICAL MASS SPECTROMETRY
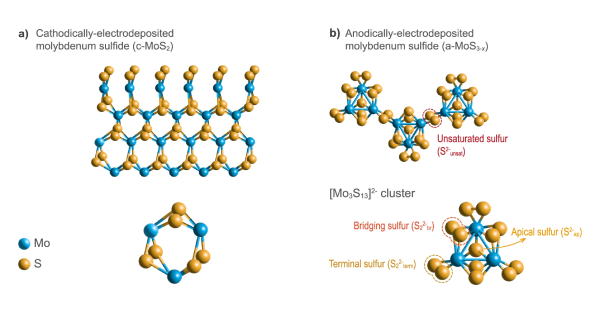
Daniel Escalera-López, Christian Iffelsberger, Matej Zlatar, Katarina Novčić, Nik Maselj, Chuyen Van Pham, Primož Jovanovič, Nejc Hodnik, Simon Thiele, Martin Pumera & Serhiy Cherevko
(2024). Allotrope-dependent activity-stability relationships of molybdenum sulfide hydrogen evolution electrocatalysts. Nature Communications volume 15, Article number: 3601 (2024) https://doi.org/10.1002/aenm.202303998

Roy, K., Rana, A., Ghosh, T. K., Heil, J. N., Roy, S., Vannoy, K. J., Tackett, B. M., Chen, M., & Dick, J. E. (2024). How Solvation Energetics Dampen the Hydrogen Evolution Reaction to Maximize Zinc Anode Stability. In Advanced Energy Materials. Wiley. https://doi.org/10.1002/aenm.202303998
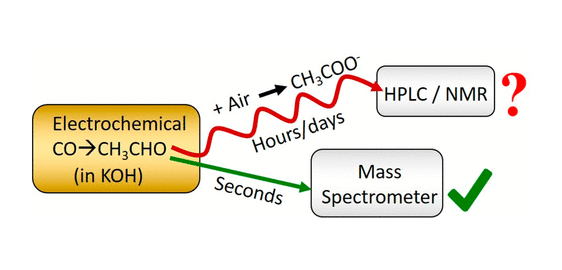
Yu Qiao, Degenhart Hochfilzer, Jakob Kibsgaard, Ib Chorkendorff, and Brian Seger (2024).
Real-Time Detection of Acetaldehyde in Electrochemical CO Reduction on Cu Single Crystal
https://pubs.acs.org/doi/10.1021/acsenergylett.3c02549
ACS Energy Letters, American Chemical Society, 3c02549
Journal of the American Chemical Society, American Chemical Society

Roy, K., Rana, A., Heil, J. N., Tackett, B. M., & Dick, J. E. (2024). For Zinc Metal Batteries, How Many Electrons go to Hydrogen Evolution? An Electrochemical Mass Spectrometry Study. doi:10.1002/anie.202319010
Angewandte Chemie International Edition, e202319010.
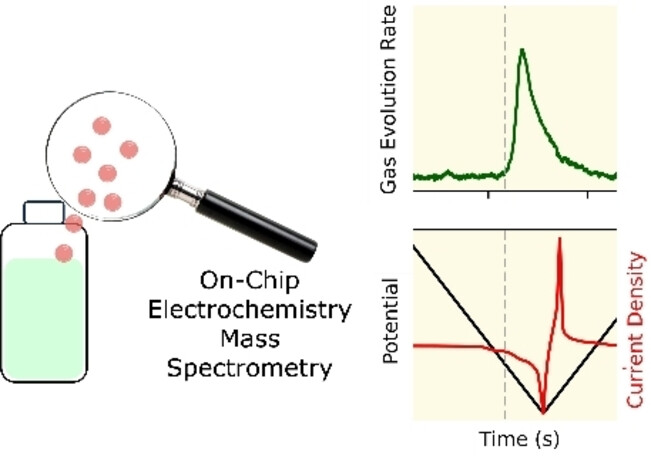
Daisy B. Thornton, Bethan J. V. Davies, Soren B. Scott, Ainara Aguadero, Mary P. Ryan, Ifan E. L. Stephens (2023).
Probing Degradation in Lithium Ion Batteries with On-Chip Electrochemistry Mass Spectrometry
https://onlinelibrary.wiley.com/doi/10.1002/anie.202315357
Angewandte Chemie, International Edition, e202315357, A Journal of the German Chemical Society
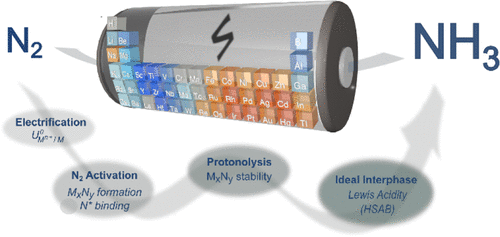
Tort, R., Bagger, A., Westhead, O., Kondo, Y., Khobnya, A., Winiwarter, A., … Stephens, I. E. L. (2023). Searching for the Rules of Electrochemical Nitrogen Fixation. ACS Catalysis, 13(22), 14513–14522. doi:10.1021/acscatal.3c03951
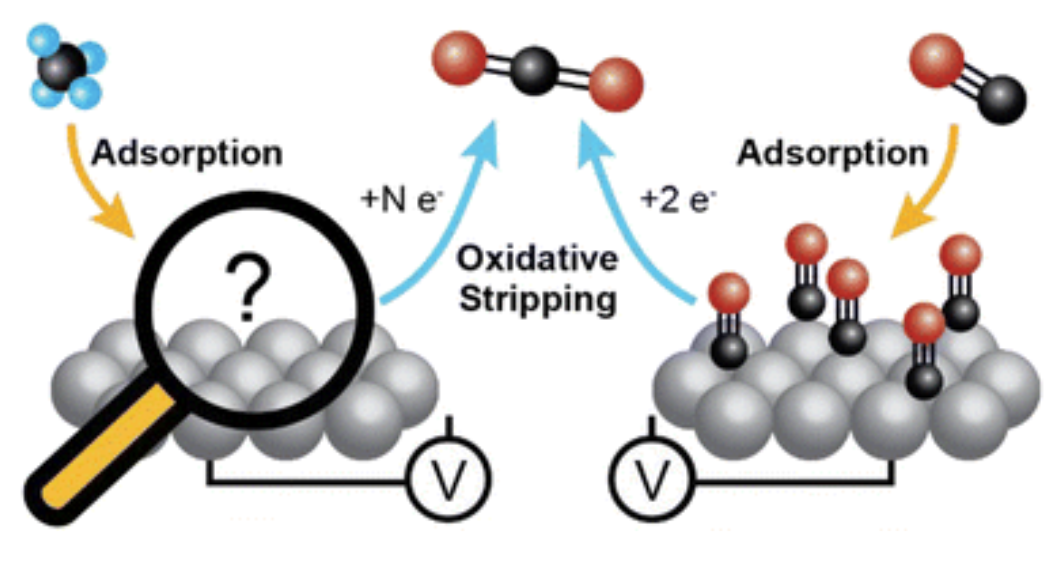
Lucky, C., Fuller, L., & Schreier, M. (2023). Determining the potential-dependent identity of methane adsorbates at Pt electrodes using EC-MS. Catal. Sci. Technol. doi:10.1039/D3CY01172K
Luka Pavko, Matija Gatalo, Tina Đukić, Francisco Ruiz-Zepeda, Angelja Kjara Surca, Martin Šala, Nik Maselj, Primož Jovanovič, Marjan Bele, Matjaž Finšgar, Boštjan Genorio, Nejc Hodnik, & Miran Gaberšček (2023). Correlating oxygen functionalities and electrochemical durability of carbon supports for electrocatalysts. Carbon, 118458. https://doi.org/10.1016/j.carbon.2023.118458
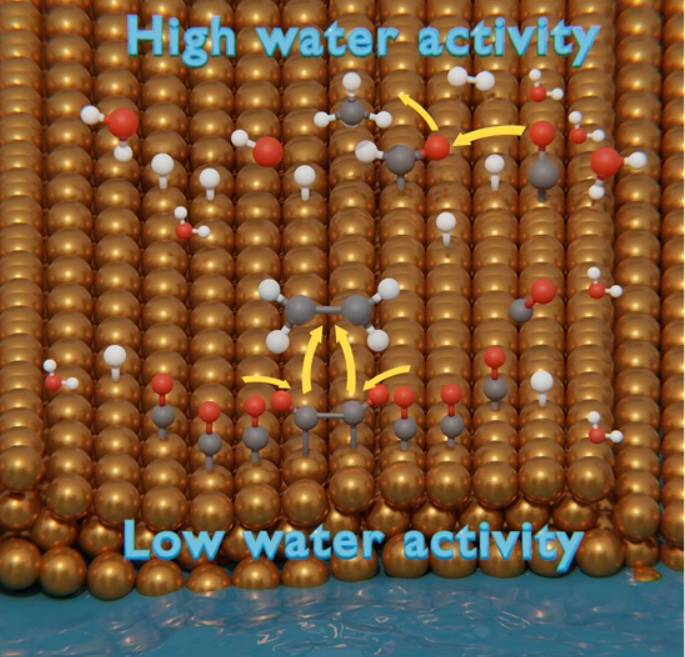
Zhang, H., Gao, J., Raciti, D. et al. Promoting Cu-catalysed CO2 electroreduction to multicarbon products by tuning the activity of H2O. Nat Catal (2023). https://doi.org/10.1038/s41929-023-01010-6
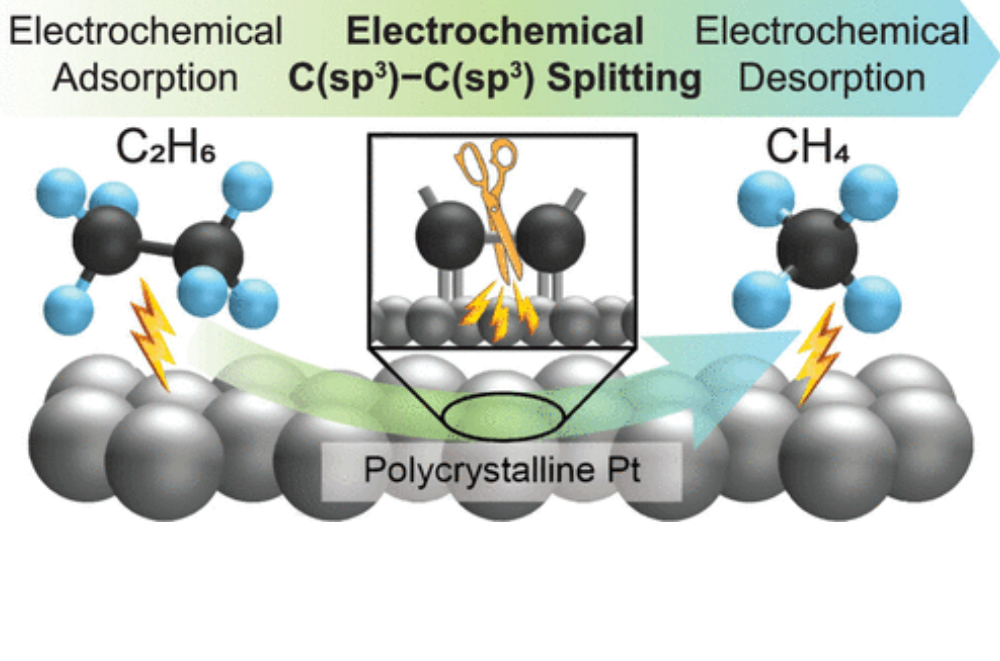
Bakshi, H. B., Lucky, C., Chen, H.-S., & Schreier, M. (2023). Electrocatalytic Scission of Unactivated C(sp3)–C(sp3) Bonds through Real-Time Manipulation of Surface-Bound Intermediates. Journal of the American Chemical Society, 145(25), 13742–13749. doi:10.1021/jacs.3c02108
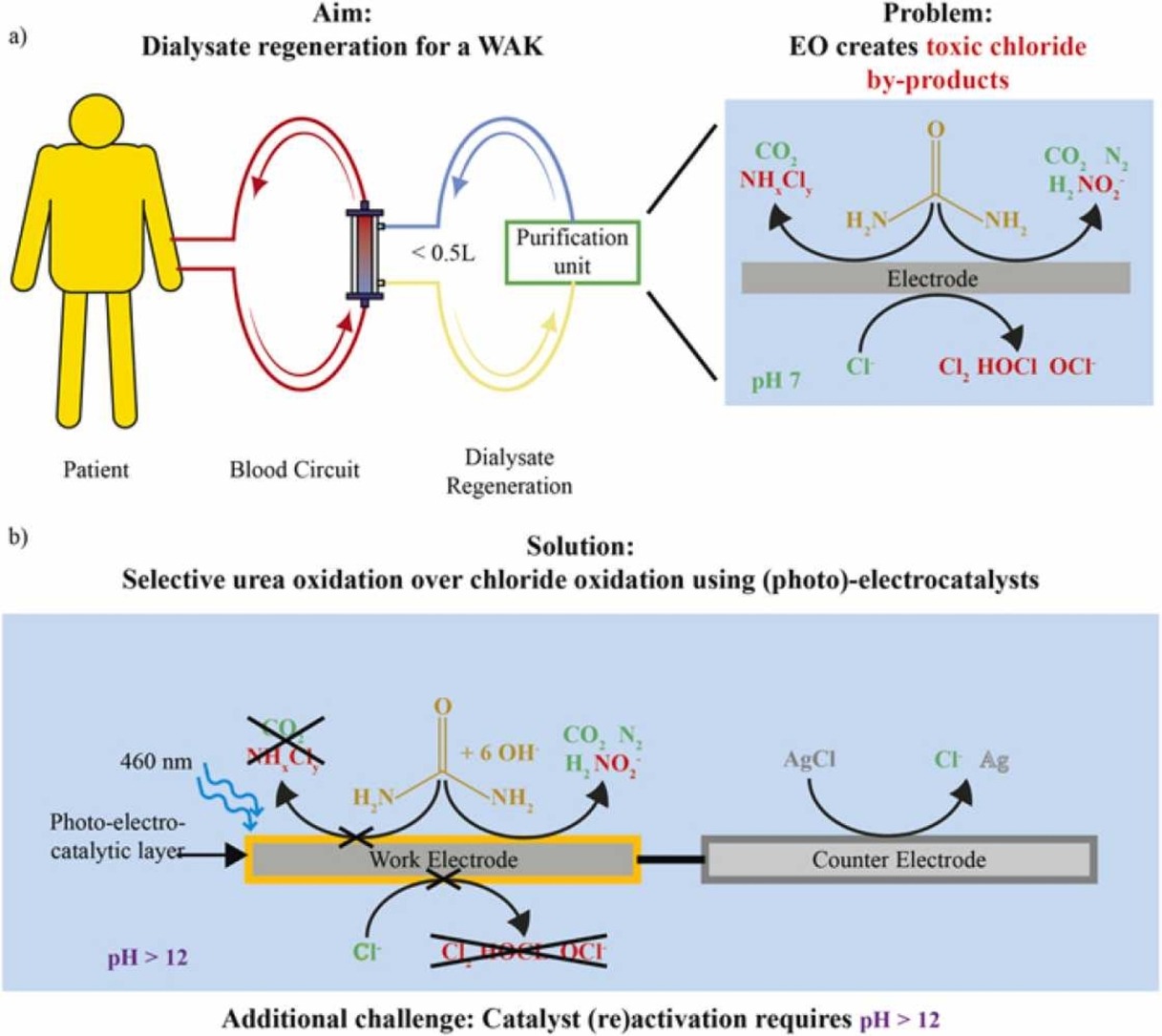
Vollenbroek, J. C., Rodriguez, A. P., Mei, B. T., Mul, G., Verhaar, M. C., Odijk, M., & Gerritsen, K. G. F. (2023). Light-driven urea oxidation for a wearable artificial kidney. In Catalysis Today (p. 114163). Elsevier BV. https://doi.org/10.1016/j.cattod.2023.114163
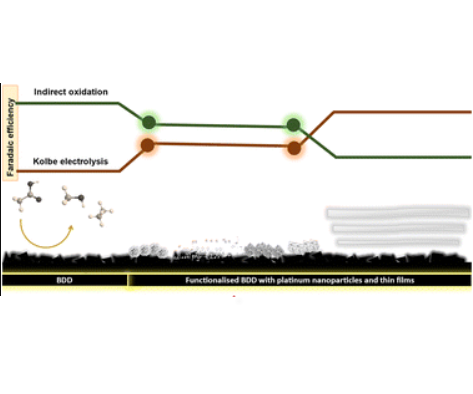
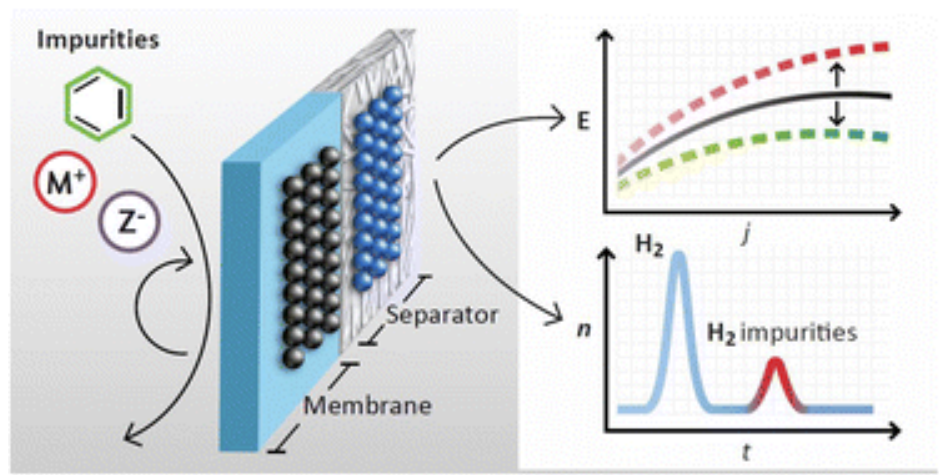
Becker, H., Murawski, J., Shinde, D. V., Stephens, I. E. L., Hinds, G., & Smith, G. (2023). Impact of impurities on water electrolysis: a review. Sustainable Energy Fuels, 7, 1565–1603. doi:10.1039/D2SE01517J
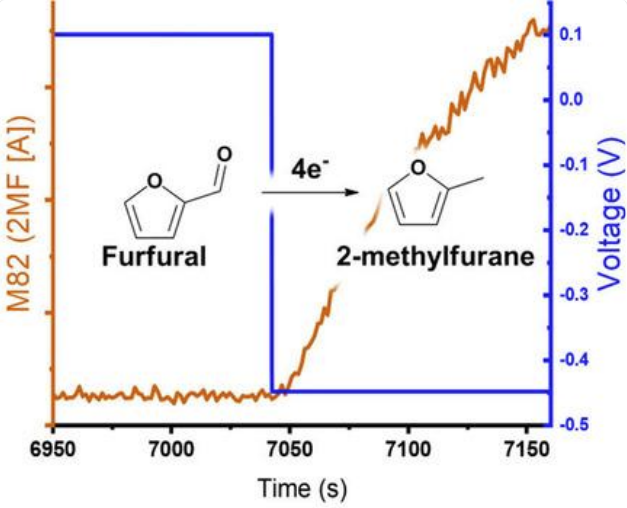
Maselj, N., Jovanovski, V., Ruiz-Zepeda, F., Finšgar, M., Klemenčič, T., Trputec, J., Kamšek, A. R., Bele, M., Hodnik, N., & Jovanovič, P. (2023). Time and Potential‐Resolved Comparison of Copper Disc and Copper Nanoparticles for Electrocatalytic Hydrogenation of Furfural. In Energy Technology (p. 2201467). Wiley. https://doi.org/10.1002/ente.202201467
Đukić, T., Pavko, L., Jovanovič, P., Maselj, N., Gatalo, M., & Hodnik, N. (2022). Stability challenges of carbon-supported Pt-nanoalloys as fuel cell oxygen reduction reaction electrocatalysts. Chem. Commun., 58, 13832–13854. https://doi.org/10.1039/D2CC05377B
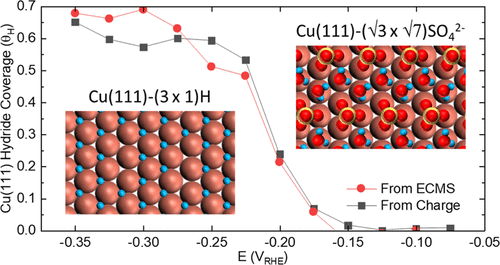
Raciti, D., & Moffat, T. P. (2022). Quantification of Hydride Coverage on Cu(111) by Electrochemical Mass Spectrometry. The Journal of Physical Chemistry C. https://doi.org/10.1021/acs.jpcc.2c06207
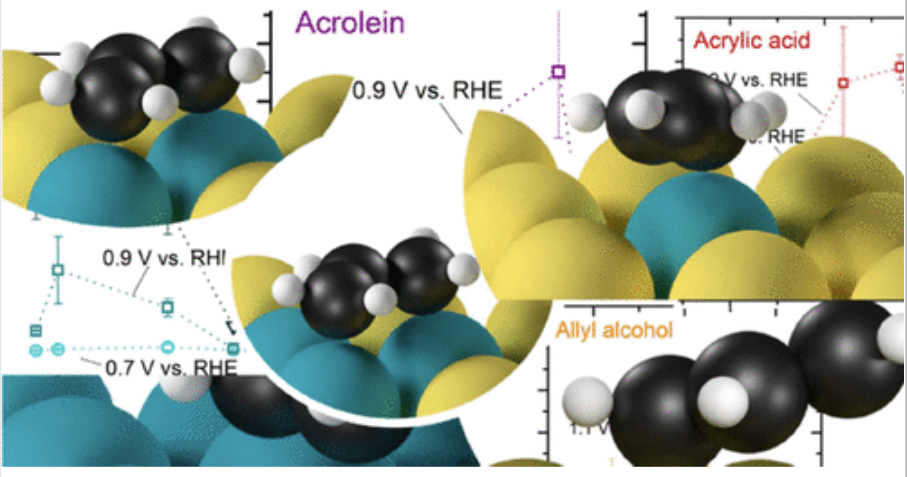
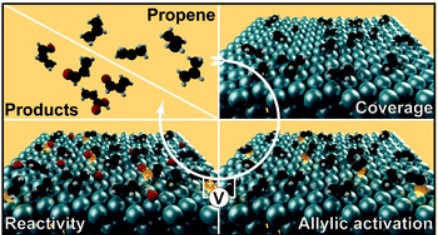
Silvioli, L., Winiwarter, A., Scott, S., Castelli, I., Moses, P., Chorkendorff, I., Seger, B., & Rossmeisl, J. (2022). Rational Catalyst Design for Higher Propene Partial Electro-oxidation Activity by Alloying Pd with Au. The Journal of Physical Chemistry C, 126. https://doi.org/10.1021/acs.jpcc.1c10095
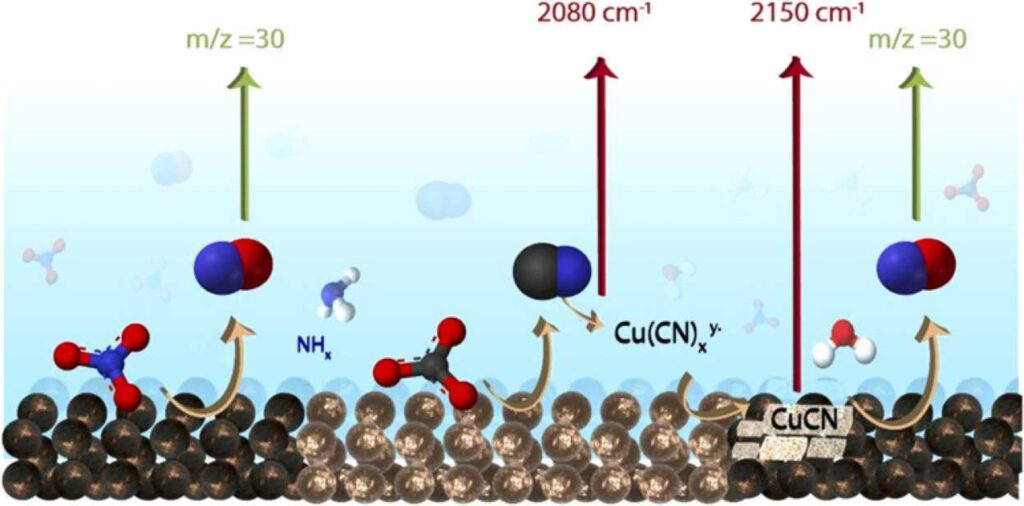
Krzywda, P. M., Paradelo Rodríguez, A., Benes, N. E., Mei, B. T., & Mul, G. (2022). Carbon-Nitrogen bond formation on Cu electrodes during CO2 reduction in NO3- solution. Applied Catalysis B: Environmental, 121512. https://doi.org/10.1016/j.apcatb.2022.121512

Oates, R. P., Murawski, J., Hor C., Shen, X., Weber, D. J., Oezaslan, M., Shaffer, M. S. P., & Stephens, I. E. L. (2022). How to Minimise Hydrogen Evolution on Carbon Based Materials?. Journal of The Electrochemical Society,169(5), 054516. https://iopscience.iop.org/article/10.1149/1945-7111/ac67f7/meta
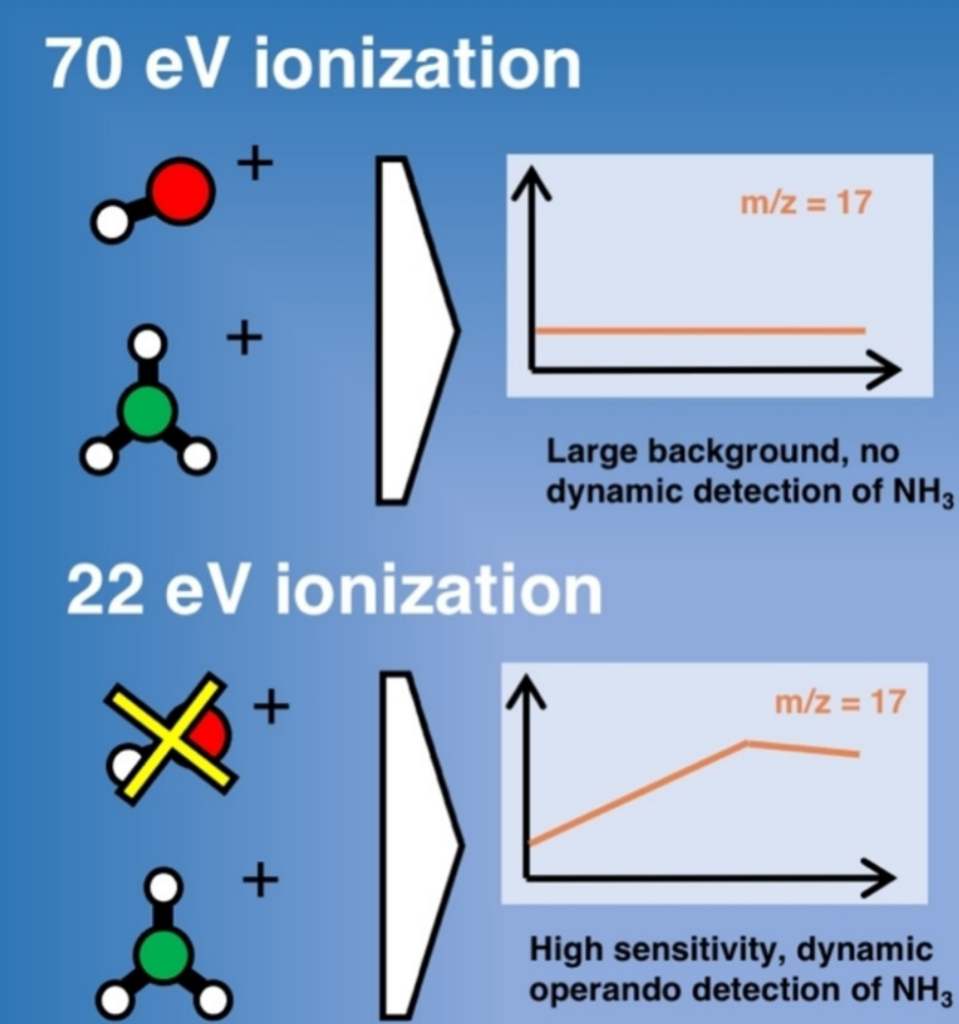
Krempl, K., Hochfilzer, D., Cavalca, F., Saccoccio, M., Kibsgaard, J., Vesborg, P., & Chorkendorff, I. (2022). Quantitative Operando Detection of Electro Synthesized Ammonia Using Mass Spectrometry. ChemElectroChem, 9(6), e202101713. https://doi.org/10.1002/celc.202101713
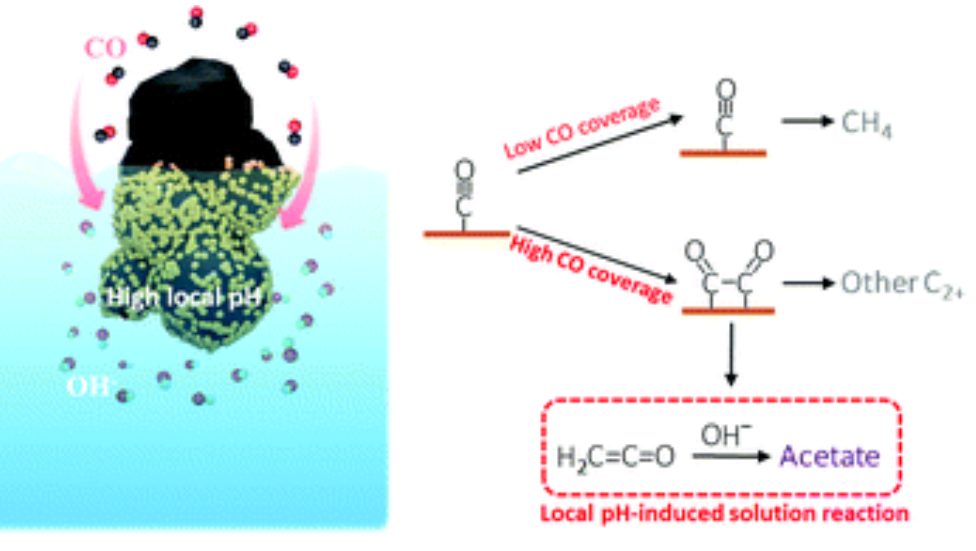
Ma, M., Deng, W., Xu, A., Hochfilzer, D., Qiao, Y., Chan, K., Chorkendorff, I., & Seger, B. (2022). Local reaction environment for selective electroreduction of carbon monoxide. Energy Environ. Sci., 15, 2470-2478. doi:10.1039/D1EE03838A
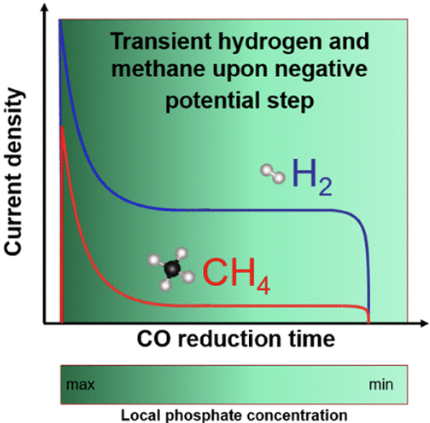
Hochfilzer, Degenhart; Xu, Aoni; Sørensen, Jakob Ejler; Needham, Julius Lucas; Krempl, Kevin; Toudahl, Karl Krøjer; et al. (2022). Transients in Electrochemical CO Reduction Explained by Mass Transport of Buffers. ACS Publications. Collection. https://doi.org/10.1021/acscatal.2c00412
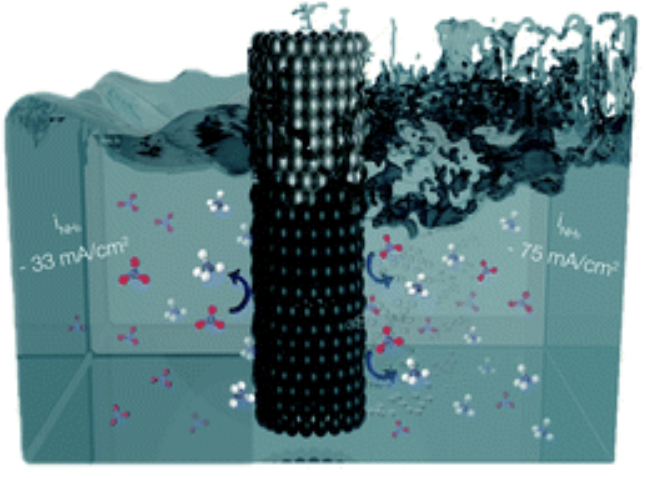
Krzywda, P. M., Paradelo Rodríguez, A., Cino, L., Benes, N. E., Mei, B. T., & Mul, G. (2022). Electroreduction of NO3− on tubular porous Ti electrodes. Catal. Sci. Technol. https://doi.org/10.1039/D2CY00289B
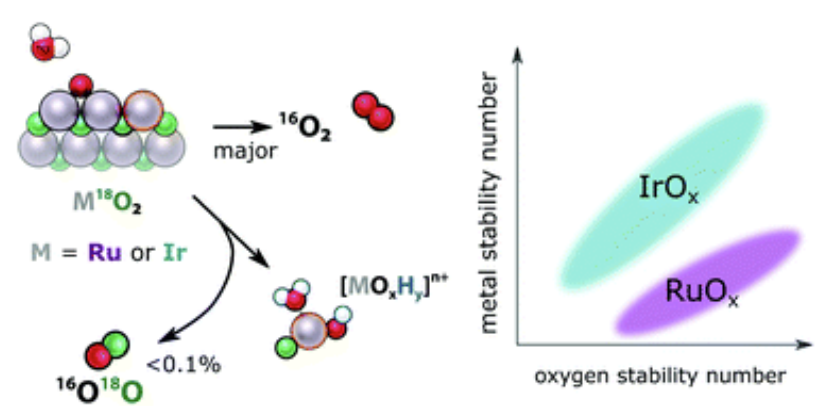
Scott, S. B., Sørensen, J. E., Rao, R. R., Moon, C., Kibsgaard, J., Shao-Horn, Y., & Chorkendorff, I. (2022). The low overpotential regime of acidic water oxidation part II: trends in metal and oxygen stability numbers. Energy Environ. Sci. https://doi.org/10.1039/D1EE03915F
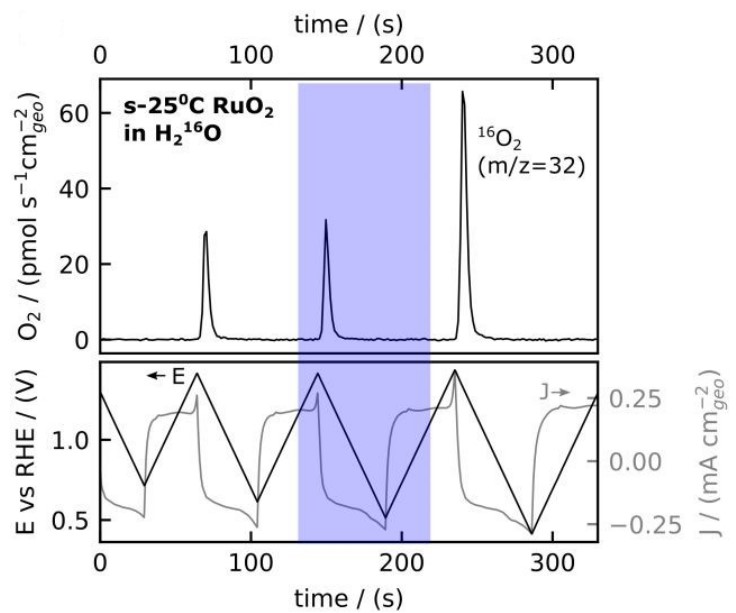
Scott, S. B., Rao, R., Moon, C., Sørensen, J. E., Kibsgaard, J., Shao-Horn, Y., & Chorkendorff, I. (2022). The low overpotential regime of acidic water oxidation part I: The importance of O2 detection. Energy Environ. Sci. https://doi.org/10.1039/D1EE03914H
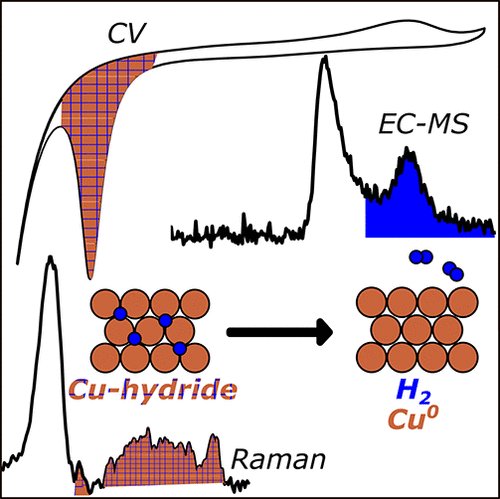
Tackett, B. M., Raciti, D., Hight Walker, A. R., & Moffat, T. P. (2021). Surface Hydride Formation on Cu(111) and Its Decomposition to Form H2 in Acid Electrolytes. The Journal of Physical Chemistry Letters, 12(44), 10936–10941. https://doi.org/10.1021/acs.jpclett.1c03131
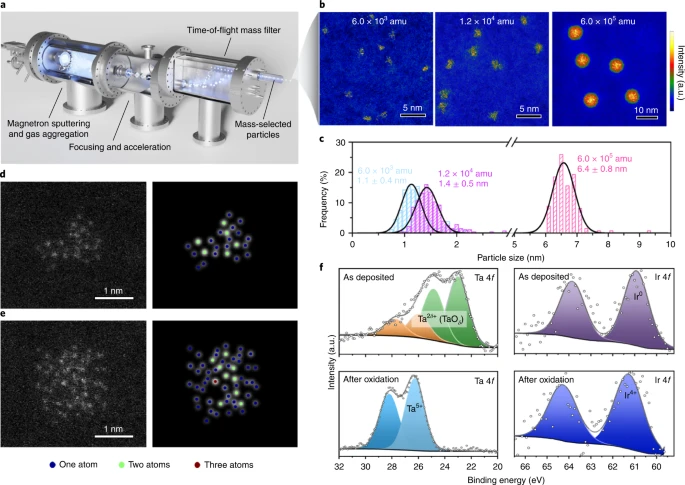
Zheng, Y.-R., Vernieres, J., Wang, Z., Zhang, K., Hochfilzer, D., Krempl, K., Liao, T.-W., Presel, F., Altantzis, T., Fatermans, J., Scott, S. B., Secher, N. M., Moon, C., Liu, P., Bals, S., van Aert, S., Cao, A., Anand, M., Nørskov, J. K., Kibsgaard, J., Chorkendorff, I. (2021). Monitoring oxygen production on mass-selected iridium–tantalum oxide electrocatalysts. Nature Energy. https://doi.org/10.1038/s41560-021-00948-w
Moriau, L. J., Hrnjić, A., Pavlišič, A., Kamšek, A. R., Petek, U., Ruiz-Zepeda, F., Šala, M., Pavko, L., Šelih, V. S., Bele, M., Jovanovič, P., Gatalo, M., & Hodnik, N. (2021). Resolving the nanoparticles’ structure-property relationships at the atomic level: a study of Pt-based electrocatalysts. In iScience (Vol. 24, Issue 2, p. 102102). Elsevier BV. https://doi.org/10.1016/j.isci.2021.102102
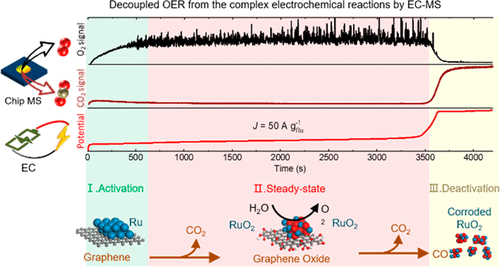
Huang, J., Scott, S. B., Chorkendorff, I., & Wen, Z. (2021). Online Electrochemistry–Mass Spectrometry Evaluation of the Acidic Oxygen Evolution Reaction at Supported Catalysts. ACS Catalysis, 11(20), 12745–12753. https://doi.org/10.1021/acscatal.1c03430
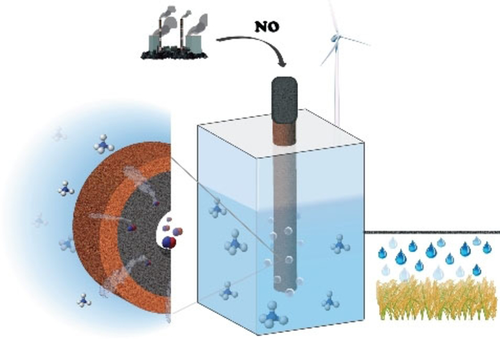
Krzywda, P. M., Paradelo Rodriguez, A., Benes, N. E., Mei, B., & Mul, G. (2021). Effect of electrolyte and electrode configuration on Cu‐catalyzed nitric oxide reduction to ammonia. ChemElectroChem. https://doi.org/10.1002/celc.202101273
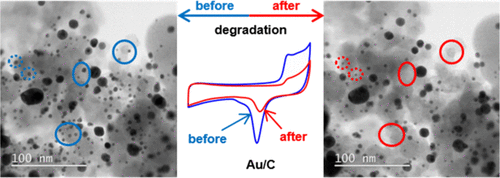
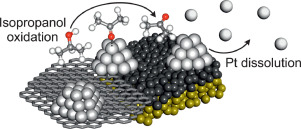
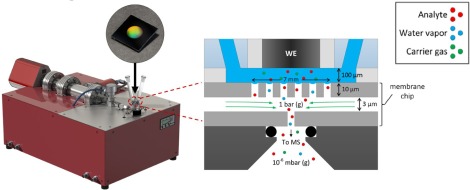
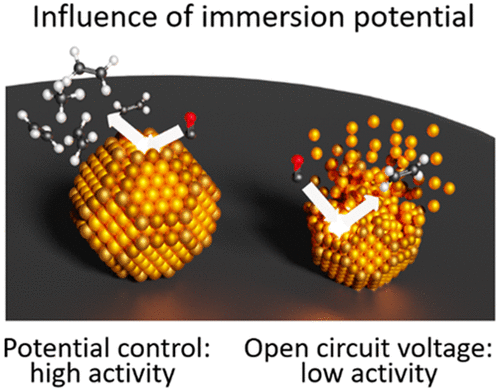
Hochfilzer, D., Sørensen, J. E., Clark, E. L., Scott, S. B., Chorkendorff, I., & Kibsgaard, J. (2021). The Importance of Potential Control for Accurate Studies of Electrochemical CO Reduction. ACS Energy Letters, 6(5). https://doi.org/10.1021/acsenergylett.1c00496
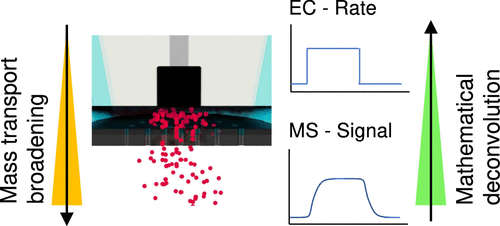
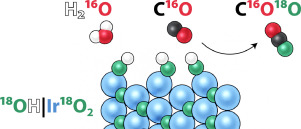
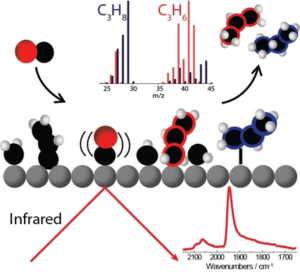
Winiwarter, A., Boyd, M. J., Scott, S. B., Higgins, D. C., Seger, B., Chorkendorff, I., & Jaramillo, T. F. (2021). CO as a Probe Molecule to Study Surface Adsorbates during Electrochemical Oxidation of Propene. ChemElectroChem, 8(1). https://doi.org/10.1002/celc.202001162
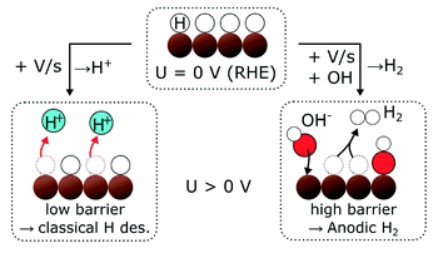
Scott, S. B., Engstfeld, A. K., Jusys, Z., Hochfilzer, D., Knøsgaard, N., Trimarco, D. B., Vesborg, P. C. K., Behm, R. J., & Chorkendorff, I. (2020). Anodic molecular hydrogen formation on Ru and Cu electrodes. Catalysis Science & Technology, 10(20). https://doi.org/10.1039/D0CY01213K